Ordre des Médecins du Brabant Wallon
Av de Tervuren 417
1150 Bruxelles
Bruxelles, le 13/10/2023
LETTRE OUVERTE
Confères de l’ordre des médecins, Dr Barroy, Melot, Bruart et autres
Ci-joint une publication récente : septembre 2023 confirmant la « pathogénicité » de la protéine Spike virale et surtout vaccinale
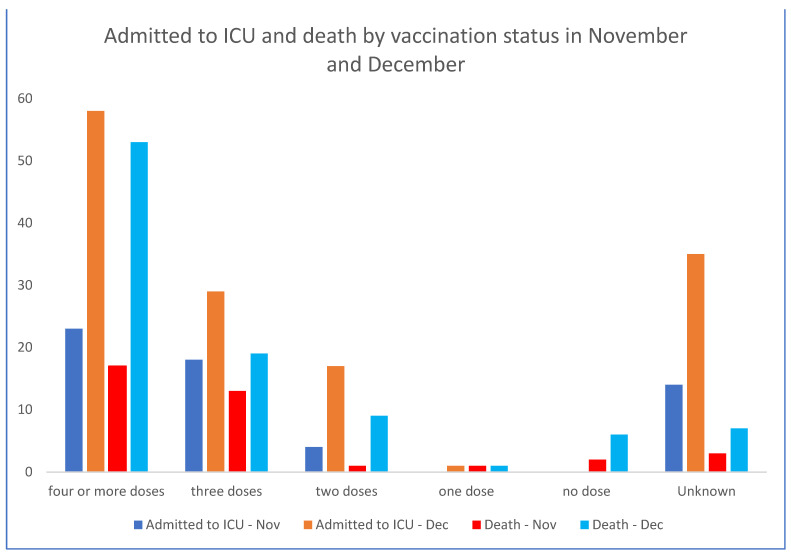
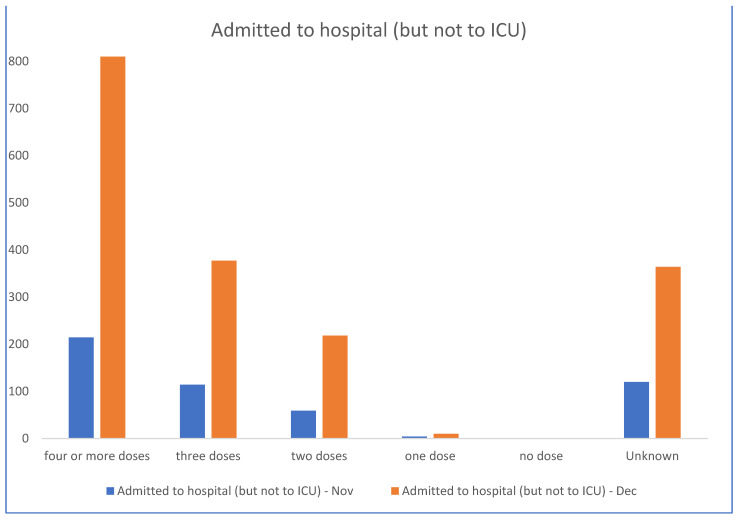
Les admissions aux soins intensifs et les décès sont directement corrélés aux nombres d’injections du pseudo vaccin
253 publications de haut vol vous satisferont-elles pour :
- enfin admettre qu’avoir poussé des collègues à injecter ce produit expérimental et hautement toxique est une énorme erreur ?
- Cesser de harceler les collègues prudents qui devant un produit génique expérimental ont été réticents à juste titre à suivre les recommandations des marchands de vaccins ?
- Par ailleurs, je vous fournis un « scoop » : comme vous l’avez constaté, l’Aviq par l’entremise du Dr Huvelle a lancé une nouvelle campagne de vaccination «toute » population : y compris femmes enceintes, enfants, adolescents alors que absolument TOUTES les études sérieuses, non sponsorisées par les marchands de vaccins démontrent leur dangerosité, inefficacité. Même Pfizer ne le recommande pas, c’est dire.
- Mortalité du « vaccin » : entre 1/800 et 1/1000 personnes piquées, ce qui donne dans une étude toute récente que vous trouverez en annexe, par extrapolation statistique (grâce aux données de pays fournissant les chiffres de décès comparés VAX/non VAX) de l’ordre de 17 millions de morts toutes causes confondues suite à l’injection de cette soupe expérimentale en phase 3-4 confondues.
- Myocardites chez les jeunes piqués : aux alentours de 1/450 : sachant que ces myocardites auto-immunes n’ont en rien le pronostic favorable des myocardites virales .
- Risque de fausses couches et malformations fœtales « explosées » suite à l’inclusion de femmes enceintes dans cette expérimentation mondiale de phase 3 et 4 confondues incluant des milliards de malheureux cobayes non consentants.
Et donc : le « scoop » ?
Une action au pénal à l’encontre de l’Aviq/le médecin fonctionnaire responsable est en cours de préparation : les avocats définiront précisément les chefs d’inculpation : défaut de prévoyance, mise en danger de la vie d’autrui, non-respect de la science etc. etc. etc. seront entre-autres évoqués.
Par la suite ou concomitamment toute personne, collègue, groupe de personnes, société scientifique, savante auront à répondre des même chefs d’inculpation.
Dans l’attente de vos réactions scientifiques et éthiques (et votre mea culpa?)
Dr Résimont Dr Kayser Dr Colignon Dr Goareger
Dr Procureur. Dr Sacré
En Anglais
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10452662/
En Français
Références
1. Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2. Solis O., Beccari A.R., Iaconis D., Talarico C., Ruiz-Bedoya C.A., Nwachukwu J.C., Cimini A., Castelli V., Bertini R., Montopoli M., et al. The SARS-CoV-2 spike protein binds and modulates estrogen receptors. Sci. Adv. 2022;8:eadd4150. doi: 10.1126/sciadv.add4150. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Kiaie S.H., Majidi Zolbanin N., Ahmadi A., Bagherifar R., Valizadeh H., Kashanchi F., Jafari R. Recent advances in mRNA-LNP therapeutics: Immunological and pharmacological aspects. J. Nanobiotechnol. 2022;20:276. doi: 10.1186/s12951-022-01478-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
4. Kariko K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
5. Therapeutic Goods Administration (TGA) FOI Reply 2389-6, p.45 Nonclinical Evaluation Report: BNT162b2 [mRNA] COVID-19 Vaccine (COMIRNATYTM). Submission No: PM-2020-05461-1-2. Sponsor: Pfizer Australia Pty Ltd. Australian Government Department of Health and Aged Care: 2021; FOI reply 2389-6. [(accessed on 7 April 2023)]; Available online: https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf
6. AstraZeneca 2.4 Nonclinical Overview AZD1222: Doc ID-004493554; MHRA: 2022-10-24-IR0751D 2021. [(accessed on 12 July 2023)]. Available online: https://icandecide.org/wp-content/uploads/2022/11/2022-10-24-IR0751D_Production_MHRA_000001-000166-166-pages.pdf
7. Geeraerts T., Guilbeau-Frugier C., Garcia C., Memier V., Raposo N., Bonneville F., Gales C., Darcourt J., Voisin S., Ribes A., et al. Immunohistologic features of cerebral venous thrombosis due to vaccine-induced immune thrombotic thrombocytopenia. Neurol. Neuroimmunol. Neuroinflamm. 2023;10:e200127. doi: 10.1212/NXI.0000000000200127. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J. Transl. Autoimmun. 2020;3:100051. doi: 10.1016/j.jtauto.2020.100051. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. Vojdani A., Vojdani E., Kharrazian D. Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases. Front. Immunol. 2021;11:617089. doi: 10.3389/fimmu.2020.617089. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
10. Reyna-Villasmil E., Caponcello M.G., Maldonado N., Olivares P., Caroccia N., Bonazzetti C., Tazza B., Carrara E., Giannella M., Tacconelli E., et al. Association of Patients’ Epidemiological Characteristics and Comorbidities with Severity and Related Mortality Risk of SARS-CoV-2 Infection: Results of an Umbrella Systematic Review and Meta-Analysis. Biomedicines. 2022;10:2437. doi: 10.3390/biomedicines10102437. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
11. Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
12. Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
13. RMIT Fact Check Did Pfizer Make a ‘Scandalous’ Admission to the European Parliament about Its COVID-19 Vaccine? ABC News. 2022. [(accessed on 6 July 2023)]. Available online: https://abc.net.au/news/2022-10-21/fact-check-pfizer-admission-transmission-european-parliament/101556606
14. Watson O.J., Barnsley G., Toor J., Hogan A.B., Winskill P., Ghani A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022;22:1293–1302. doi: 10.1016/S1473-3099(22)00320-6.[PMC free article] [PubMed] [CrossRef] [Google Scholar]
15. Roussel Y., Giraud-Gatineau A., Jimeno M.T., Rolain J.M., Zandotti C., Colson P., Raoult D. SARS-CoV-2: Fear versus data. Int. J. Antimicrob. Agents. 2020;55:105947. doi: 10.1016/j.ijantimicag.2020.105947. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
16. Ioannidis J.P.A., Cripps S., Tanner M.A. Forecasting for COVID-19 has failed. Int. J. Forecast. 2022;38:423–438. doi: 10.1016/j.ijforecast.2020.08.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
17. Rid A., Lipsitch M., Miller F.G. The Ethics of Continuing Placebo in SARS-CoV-2 Vaccine Trials. JAMA. 2021;325:219–220. doi: 10.1001/jama.2020.25053. [PubMed] [CrossRef] [Google Scholar]
18. WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Evaluation. Krause P.R., Fleming T.R., Longini I.M., Peto R., Beral V., Bhargava B., Cravioto A., Cramer J.P., Ellenberg S.S., et al. Placebo-Controlled Trials of Covid-19 Vaccines—Why We Still Need Them. N. Engl. J. Med. 2021;384:e2. doi: 10.1056/NEJMp2033538. [PubMed] [CrossRef] [Google Scholar]
19. Control Group Cooperative The Covid Vaccine Study. 2021. [(accessed on 3 July 2023)]. Available online: https://www.vcgwiki.com/the-covid-vaccine-study
20. Verkerk R., Kathrada N., Plothe C., Lindley K. Self-selected COVID-19 “unvaccinated” cohort reports favorable health outcomes and unjustified discrimination in global survey. Int. J. Vaccine Theory Pract. Res. 2022;2:321–354. doi: 10.56098/ijvtpr.v2i2.43. [CrossRef] [Google Scholar]
21. NSW Health NSW Respiratory Surveillance Report—Week Ending 31 December 2022. [(accessed on 10 July 2023)];2022 Available online: https://www.health.nsw.gov.au/Infectious/covid-19/Documents/weekly-covid-overview-20221231.pdf
22. Therapeutic Goods Administration (TGA) COVID-19 vaccines regulatory status. Australian Government Department of Health and Aged Care: Tga.gov.au. [(accessed on 7 April 2023)];2023 Available online: https://www.tga.gov.au/products/covid-19/covid-19-vaccines/covid-19-vaccine-provisional-registrations
23. Therapeutic Goods Administration (TGA) TGA provisionally approves Novavax (Biocelect Pty Ltd’s COVID-19 vaccine NUVAXOVID. Australian Government Department of Health and Aged Care: Tga.gov.au. [(accessed on 7 April 2023)];2023 Available online: https://www.tga.gov.au/news/media-releases/tga-provisionally-approves-novavax-biocelect-pty-ltds-covid-19-vaccine-nuvaxovid
24. Senate Committee: Community Affairs Committee . Answers to Questions on Notice, Outcome: 1—Health Policy, Access and Support, 2022–2023 Budget Estimates October and November. Australian Federal Parliament; Canberra, Australia: 2022. [Google Scholar]
25. Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
26. Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
27. Cuffari B. What are Spike Proteins? News-Medical.Net. 2021. [(accessed on 26 April 2023)]. Available online: https://www.news-medical.net/health/What-are-Spike-Proteins.aspx
28. Carnell G.W., Ciazynska K.A., Wells D.A., Xiong X., Aguinam E.T., McLaughlin S.H., Mallery D., Ebrahimi S., Ceron-Gutierrez L., Asbach B., et al. SARS-CoV-2 Spike Protein Stabilized in the Closed State Induces Potent Neutralizing Responses. J. Virol. 2021;95:e0020321. doi: 10.1128/JVI.00203-21. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
29. Seneff S., Kyriakopoulos A.M., Nigh G., McCullough P.A. A Potential Role of the Spike Protein in Neurodegenerative Diseases: A Narrative Review. Cureus. 2023;15:e34872. doi: 10.7759/cureus.34872. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
30. Changeux J.P., Amoura Z., Rey F.A., Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Comptes Rendus Biol. 2020;343:33–39. doi: 10.5802/crbiol.8. [PubMed] [CrossRef] [Google Scholar]
31. Nirthanan S. Snake three-finger α-neurotoxins and nicotinic acetylcholine receptors: Molecules, mechanisms and medicine. Biochem. Pharmacol. 2020;181:114168. doi: 10.1016/j.bcp.2020.114168. [PubMed] [CrossRef] [Google Scholar]
32. Farsalinos K., Niaura R., Le Houezec J., Barbouni A., Tsatsakis A., Kouretas D., Vantarakis A., Poulas K. Editorial: Nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol. Rep. 2020;7:658–663. doi: 10.1016/j.toxrep.2020.04.012. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
33. O’Brien B.C.V., Weber L., Hueffer K., Weltzin M.M. SARS-CoV-2 spike ectodomain targets α7 nicotinic acetylcholine receptors. J. Biol. Chem. 2023;299:104707. doi: 10.1016/j.jbc.2023.104707. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
34. ACROBiosystems An Overview of Different COVID-19 Vaccines. ACROBiosystems Insights, 2021. An Overview of Different COVID-19 Vaccines—ACROBiosystems. [(accessed on 7 April 2023)]. Available online: https://www.acrobiosystems.com/A1374-An-Overview-of-Different-COVID-19-Vaccines.html
35. Wikipedia List of COVID-19 vaccine authorizations. 2023. [(accessed on 7 April 2023)]. Available online: https://en.wikipedia.org/wiki/List_of_COVID-19_vaccine_authorizations
36. U.S. Food and Drug Administration (FDA) FDA News Release: FDA Approves First-of-Its Kind Targeted RNA-Based Therapy to Treat a Rare Disease. FDA Newsroom FDA.gov.au. [(accessed on 7 April 2023)];2018 Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-targeted-rna-based-therapy-treat-rare-disease
37. Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597:318–324. doi: 10.1038/d41586-021-02483-w.[PubMed] [CrossRef] [Google Scholar]
38. McCann N., O’Connor D., Lambe T., Pollard A.J. Viral vector vaccines. Curr. Opin. Immunol. 2022;77:102210. doi: 10.1016/j.coi.2022.102210. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
39. Altman P.M., Rowe J., Hoy W., Brady G., Lefringhausen A., Cosford R., Wauchope B. Did National Security Imperatives Compromise COVID-19 Vaccine Safety? Trial Site News. 2022. [(accessed on 9 June 2023)]. Available online: https://www.trialsitenews.com/a/did-national-security-imperatives-compromise-covid-19-vaccine-safety-adfea242
40. Lalani H.S., Nagar S., Sarpatwari A., Barenie R.E., Avorn J., Rome B.N., Kesselheim A.S. US public investment in development of mRNA covid-19 vaccines: Retrospective cohort study. BMJ. 2023;380:e073747. doi: 10.1136/bmj-2022-073747. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
41. McCullough P. America’s long, expensive, and deadly love affair with mRNA. In Courageous Discourse, Substack.com: 2023. [(accessed on 15 March 2023)]. Available online: https://petermcculloughmd.substack.com/p/americas-long-expensive-and-deadly
42. Turni C., Lefringhausen A. Covid-19 vaccines—An Australian Review. J. Clin. Exp. Immunol. 2022;7:491–508. [Google Scholar]
43. Ndeupen S., Qin Z., J.sen S., Bouteau A., Estanbouli H., Igyártó B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24:103479. doi: 10.1016/j.isci.2021.103479. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
44. Wick P., Malek A., Manser P., Meili D., Maeder-Althaus X., Diener L., Diener P.A., Zisch A., Krug H.F., von Mandach U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010;118:432–436. doi: 10.1289/ehp.0901200. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
45. Zhou Y., Peng Z., Seven E.S., Leblanc R.M. Crossing the blood-brain barrier with nanoparticles. J. Control Release. 2018;270:290–303. doi: 10.1016/j.jconrel.2017.12.015. [PubMed] [CrossRef] [Google Scholar]
46. Japanese Pharmaceuticals and Medical Devices Agency (PMDA) SARS-CoV-2 mRNA Vaccine (BNT162, PF-07302048) 2021. [(accessed on 7 April 2023)]. Available online: https://www.pmda.go.jp/drugs/2021/P20210212001/672212000_30300AMX00231_I100_1.pdf
47. Judicial Watch Pfizer/BioNTech Study Found Lipid Nanoparticles Materials Outside Injection Site in Test Animals. judicialwatch.org. 2022. [(accessed on 12 July 2023)]. Available online: https://www.judicialwatch.org/nanoparticles-materials-outside-injection-site/
48. Di J., Du Z., Wu K., Jin S., Wang X., Li T., Xu Y. Biodistribution and Non-linear Gene Expression of mRNA LNPs Affected by Delivery Route and Particle Size. Pharm. Res. 2022;39:105–114. doi: 10.1007/s11095-022-03166-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
49. Morais P., Adachi H., Yu Y.T. The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Front. Cell. Dev. Biol. 2021;9:789427. doi: 10.3389/fcell.2021.789427. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
50. Fertig T.E., Chitoiu L., Marta D.S., Ionescu V.S., Cismasiu V.B., Radu E., Angheluta G., Dobre M., Serbanescu A., Hinescu M.E., et al. Vaccine mRNA can be detected in blood at 15 days post-vaccination. Biomedicines. 2022;10:1538. doi: 10.3390/biomedicines10071538. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
51. Castruita J.A.S., Schneider U.V., Mollerup S., Leineweber T.D., Weis N., Bukh J., Pedersen M.S., Westh H. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS. 2023;131:128–132. doi: 10.1111/apm.13294. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
52. Ogata A.F., Cheng C.-A., Desjardins M., Senussi Y., Sherman A.C., Powell M., Novack L., Von S., Li X., Baden L.R., et al. Circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin. Infect. Dis. 2021;74:715–718. doi: 10.1093/cid/ciab465. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
53. Röltgen K., Nielsen S.C.A., Silva O., Younes S.F., Zaslavsky M., Costales C., Yang F., Wirz O.F., Solis D., Hoh R.A., et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185:1025–1040.e14. doi: 10.1016/j.cell.2022.01.018. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
54. Yonker L.M., Swank Z., Bartsch Y.C., Burns M.D., Kane A., Boribong B.P., Davis J.P., Loiselle M., Novak T., Senussi Y., et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023;147:867–876. doi: 10.1161/CIRCULATIONAHA.122.061025. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
55. Jikomes N. Pseudouridine, mRNA Vaccines & Spike Protein Persistence. In Mind & Matter with Nick Jikomes, mindandmatter.substack.com. 2022. [(accessed on 7 April 2023)]. Available online: https://mindandmatter.substack.com/p/pseudouridine-mrna-vaccines-and-spike
56. Yong S.J. mRNA Vaccine Stays Active in the Body Longer than Expected, New Data Shows. But it isn’t Dangerous. In Microbial Instincts, Medium.com. 2022. [(accessed on 7 April 2023)]. Available online: https://medium.com/microbial-instincts/mrna-vaccine-stays-active-in-the-body-longer-than-expected-new-data-shows-but-it-isnt-harmful-aaa40544bc06
57. Bansal S., Perincheri S., Fleming T., Poulson C., Brian T., Bremner M.R., Mohanakumar T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021;207:2405–2410. doi: 10.4049/jimmunol.2100637. [PubMed] [CrossRef] [Google Scholar]
58. Yamamoto M., Kase M., Sano H., Kamijima R., Sano S. Persistent varicella zoster virus infection following mRNA COVID-19 vaccination was associated with the presence of encoded spike protein in the lesion. J. Cutan. Immunol. Allergy. 2023;6:18–23. doi: 10.1002/cia2.12278. [CrossRef] [Google Scholar]
59. Maugeri M., Nawaz M., Papadimitriou A. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019;10:4333. doi: 10.1038/s41467-019-12275-6. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
60. Wang R., Huang K. CCL11 increases the proportion of CD4+CD25+Foxp3+ Treg cells and the production of IL 2 and TGF β by CD4+ T cells via the STAT5 signaling pathway. Mol. Med. Rep. 2020;21:2522–2532. doi: 10.3892/mmr.2020.11049. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
61. Segalla G. Chemical-physical criticality and toxicological potential of lipid nanomaterials contained in a COVID-19 mRNA vaccine. Int. J. Vaccine Theory Pract. Res. Inj. Causes Treat. 2023;3:787–817. doi: 10.56098/ijvtpr.v3i1.68. [CrossRef] [Google Scholar]
62. European Parliament Parliamentary question P-005690/2021: Excipients ALC-0315 and ALC-0159. Priority Question for Written Answer to the Commission, Rule 138, Guido Reil (ID); European Parliament Europarl.europa.eu 2021. [(accessed on 4 June 2023)]. Available online: https://www.europarl.europa.eu/doceo/document/P-9-2021-005690_EN.html
63. Bushmanova S.V., Ivanov A.O., Buyevich Y.U. Physica A: Statistical Mechanics and Its Applications. Volume 202. Elsevier; Amsterdam, The Netherlands: 1994. The effect of an electrolyte on phase separation in colloids; pp. 175–195. [Google Scholar]
64. Poon W., Zhang Y.N., Ouyang B., Kingston B.R., Wu J.L.Y., Wilhelm S., Chan W.C.W. Elimination Pathways of Nanoparticles. ACS Nano. 2019;13:5785–5798. doi: 10.1021/acsnano.9b01383. [PubMed] [CrossRef] [Google Scholar]
65. Trougakos I.P., Terpos E., Alexopoulos H., Politou M., Paraskevis D., Scorilas A., Kastritis E., Andreakos E., Dimopoulos M.A. COVID-19 mRNA vaccine-induced adverse effects: Unwinding the unknowns. Trends Mol. Med. 2022;28:800–802. doi: 10.1016/j.molmed.2022.07.008. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
66. Halma M.T., Rose J., Lawrie T. The novelty of mRNA viral vaccines and potential harms: A scoping review. J. 2023;6:220–235. doi: 10.3390/j6020017. [CrossRef] [Google Scholar]
67. Yamamoto K. Adverse effects of Covid-19 vaccines and measures to prevent them. Virol. J. 2022;19:100. doi: 10.1186/s12985-022-01831-0. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
68. Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., Maurus D., Schwarck-Kokarakis D., Kuhn A.N., Omokoko T., et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [PubMed] [CrossRef] [Google Scholar]
69. Doener F., Hong H.S., Meyer I., Tadjalli-Mehr K., Daehling A., Heidenreich R., Koch S.D., Fotin-Mleczek M., Gnad-Vogt U. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine. 2019;37:1819–1826. doi: 10.1016/j.vaccine.2019.02.024. [PubMed] [CrossRef] [Google Scholar]
70. Anttila V., Saraste A., Knuuti J., Jaakkola P., Hedman M., Svedlund S., Lagerström-Fermér M., Kjaer M., Jeppsson A., Gan L.M. Synthetic mRNA Encoding VEGF-A in Patients Undergoing Coronary Artery Bypass Grafting: Design of a Phase 2a Clinical Trial. Mol. Ther. Methods Clin. Dev. 2020;18:464–472. doi: 10.1016/j.omtm.2020.05.030. [PMC free article][PubMed] [CrossRef] [Google Scholar]
71. Crescioli S., Correa I., Karagiannis P., Davies A.M., Sutton B.J., Nestle F.O., Karagiannis S.N. IgG4 Characteristics and Functions in Cancer Immunity. Curr. Allergy Asthma Rep. 2016;16:7. doi: 10.1007/s11882-015-0580-7. [PMC free article][PubMed] [CrossRef] [Google Scholar]
72. Schlaudecker E.P., McNeal M.M., Dodd C.N., Ranz J.B., Steinhoff M.C. Pregnancy modifies the antibody response to trivalent influenza immunization. J. Infect. Dis. 2012;206:1670–1673. doi: 10.1093/infdis/jis592. [PubMed] [CrossRef] [Google Scholar]
73. Zhang X., Lu H., Peng L., Zhou J., Wang M., Li J., Liu Z., Zhang W., Zhao Y., Zeng X., et al. The role of PD-1/PD-Ls in the pathogenesis of IgG4-related disease. Rheumatology. 2022;61:815–825. doi: 10.1093/rheumatology/keab360. [PubMed] [CrossRef] [Google Scholar]
74. Medsafe Alert Communication: Myocarditis and Pericarditis have been Reported with Nuvaxovid (Novavax COVID-19 vaccine). New Zealand Medicines and Medical Devices Safety Authority: Medsafe.govt.nz. [(accessed on 7 April 2023)];2022 Available online: https://www.medsafe.govt.nz/safety/Alerts/nuvaxovid-myocarditis.asp
75. AstraZeneca 2.4 Nonclinical Overview AZD1222: Doc ID-004365565; MHRA: 2022-10-24-IR0751D 2020. [(accessed on 12 July 2023)]. Available online: https://icandecide.org/wp-content/uploads/2022/11/2022-10-24-IR0751D_Production_MHRA_000001-000166-166-pages.pdf
76. Biotech B. Covaxin—India’s First Indigenous COVID-19 Vaccine. 2022. [(accessed on 8 April 2023)]. Available online: https://www.bharatbiotech.com/covaxin.html
77. Sinovac Overview of CoronaVac. 2021. [(accessed on 8 April 2023)]. Available online: http://www.coronavac.cn/
78. Vaxine COVID-19 Project. 2022. [(accessed on 11 June 2023)]. Available online: https://vaxine.net/projects/
79. CinnaGen SpikoGen: Recombinant COVID-19 Vaccine. 2022. [(accessed on 11 June 2023)]. Available online: https://www.cinnagen.com/Product.aspx?t=2&l=1&Id=607
80. Tabarsi P., Anjidani N., Shahpari R., Mardani M., Sabzvari A., Yazdani B., Roshanzamir K., Bayatani B., Taheri A., Petrovsky N., et al. Safety and immunogenicity of SpikoGen®, an Advax-CpG55.2-adjuvanted SARS-CoV-2 spike protein vaccine: A phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations. Clin. Microbiol. Infect. 2022;28:1263–1271. doi: 10.1016/j.cmi.2022.04.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
81. Tabarsi P., Anjidani N., Shahpari R., Roshanzamir K., Fallah N., Andre G., Petrovsky N., Barati S. Immunogenicity and safety of SpikoGen®, an adjuvanted recombinant SARS-CoV-2 spike protein vaccine as a homologous and heterologous booster vaccination: A randomized placebo-controlled trial. Immunology. 2022;167:340–353. doi: 10.1111/imm.13540. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
82. Tabarsi P., Anjidani N., Shahpari R., Mardani M., Sabzvari A., Yazdani B., Kafi H., Fallah N., Ebrahimi A., Taheri A., et al. Evaluating the efficacy and safety of SpikoGen®, an Advax-CpG55.2-adjuvanted severe acute respiratory syndrome coronavirus 2 spike protein vaccine: A phase 3 randomized placebo-controlled trial. Clin. Microbiol. Infect. 2023;29:215–220. doi: 10.1016/j.cmi.2022.09.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
83. Scendoni R., Cingolani M. What do we know about pathological mechanism and pattern of lung injury related to SARS-CoV-2 Omicron variant? Diagn. Pathol. 2023;18:18. doi: 10.1186/s13000-023-01306-y. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
84. Department of Health and Aged Care. Comirnaty (Pfizer). Australian Government [(accessed on 11 June 2023)];2022 Available online: https://www.health.gov.au/our-work/covid-19-vaccines/our-vaccines/pfizer
85. Therapeutic Goods Administration (TGA) Moderna COVID-19 Bivalent (SPIKEVAX Bivalent Original/Omicron BA.4-5) Booster Dose Vaccine. Australian Government Department of Health and Aged Care. [(accessed on 11 June 2023)];2023 Available online: https://www.tga.gov.au/products/covid-19/covid-19-vaccines/covid-19-vaccine-provisional-registrations/moderna-covid-19-bivalent-spikevax-bivalent-originalomicron-ba4-5-booster-dose-vaccine
86. Cosentino M., Marino F. Understanding the pharmacology of COVID-19 mRNA vaccines: Playing dice with the spike? Int. J. Mol. Sci. 2022;23:10881. doi: 10.3390/ijms231810881. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
87. Singh H.N., Singh A.B. S2 Subunit of SARS-nCoV-2 Interacts with Tumor Suppressor Protein p53 and BRCA: An In Silico Study. Transl. Oncol. 2020;13:100814. doi: 10.1016/j.tranon.2020.100814. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
88. Barreda D., Santiago C., Rodríguez J.R., Rodríguez J.F., Casasnovas J.M., Mérida I., Ávila-Flores A. SARS-CoV-2 Spike Protein and Its Receptor Binding Domain Promote a Proinflammatory Activation Profile on Human Dendritic Cells. Cells. 2021;10:3279. doi: 10.3390/cells10123279. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
89. Suzuki Y.J., Nikolaienko S.I., Dibrova V.A., Dibrova Y.V., Vasylyk V.M., Novikov M.Y., Shults N.V., Gychka S.G. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vascul. Pharmacol. 2021;137:106823. doi: 10.1016/j.vph.2020.106823. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
90. Colunga Biancatelli R.M.L., Solopov P.A., Sharlow E.R., Lazo J.S., Marik P.E., Catravas J.D. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021;321:L477–L484. doi: 10.1152/ajplung.00223.2021. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
91. McKernan K., Kyriakopoulos A.M., McCullough P.A. Differences in vaccine and SARS-CoV-2 replication derived mRNA: Implications for cell biology and future disease. OSF Preprints. 2021 doi: 10.31219/osf.io/bcsa6. [CrossRef] [Google Scholar]
92. Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., Zhang Y., Yin Q., Cho Y., Andrade L., et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021;128:1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
93. Nadwa E.H., Al-Kuraishy H.M., Al-Gareeb A.I., Elekhnawy E., Albogami S.M., Alorabi M., Batiha G.E., De Waard M. Cholinergic dysfunction in COVID-19: Frantic search and hoping for the best. Naunyn Schmiedebergs Arch. Pharmacol. 2023;396:453–468. doi: 10.1007/s00210-022-02346-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
94. Alexandris N., Lagoumintzis G., Chasapis C.T., Leonidas D.D., Papadopoulos G.E., Tzartos S.J., Tsatsakis A., Eliopoulos E., Poulas K., Farsalinos K. Nicotinic cholinergic system and COVID-19: Toxicol. Rep. 2021;8:73–83. doi: 10.1016/j.toxrep.2020.12.013. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
95. Hollenhorst M.I., Krasteva-Christ G. Nicotinic Acetylcholine Receptors in the Respiratory Tract. Molecules. 2021;26:6097. doi: 10.3390/molecules26206097. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
96. Al-Kuraishy H.M., Al-Gareeb A.I., Qusti S., Alshammari E.M., Gyebi G.A., Batiha G.E. Covid-19-Induced Dysautonomia: A Menace of Sympathetic Storm. ASN Neuro. 2021;13:17590914211057635. doi: 10.1177/17590914211057635. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
97. Patterson B.K., Seethamraju H., Dhody K., Corley M.J., Kazempour K., Lalezari J., Pang A.P.S., Sugai C., Mahyari E., Francisco E.B., et al. CCR5 inhibition in critical COVID-19 patients decreases inflammatory cytokines, increases CD8 T-cells, and decreases SARS-CoV2 RNA in plasma by day 14. Int. J. Infect. Dis. 2021;103:25–32. doi: 10.1016/j.ijid.2020.10.101.[CrossRef] [Google Scholar]
98. Henrion-Caude A. Spikopathy: The Pathology of the Spike Protein. Conference Presentation, General Assembly Meeting, World Council for Health. worldcouncilforhealth.org. 2021. [(accessed on 7 April 2023)]. Available online: https://worldcouncilforhealth.org/multimedia/alexandra-henrion-caude-france-spikopathy/
99. Anderson S. CBER Plans for Monitoring COVID-19 Vaccine Safety and Effectiveness. U.S. Food & Drug Administration (FDA): Vaccines and Related Biological Products Advisory Committee meeting. [(accessed on 7 April 2023)];2020 Available online: https://www.fda.gov/media/143557/download
100. React19 3400+ COVID Vaccine Publications and Case Reports. 2022. [(accessed on 11 June 2023)]. Available online: https://react19.org/1250-covid-vaccine-reports/
101. Avolio E., Carrabba M., Milligan R., Kavanagh Williamson M., Beltrami A.P., Gupta K., Elvers K.T., Gamez M., Foster R.R., Gillespie K., et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: A potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. 2021;135:2667–2689. doi: 10.1042/CS20210735. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
102. Cao X., Nguyen V., Tsai J., Gao C., Tian Y., Zhang Y., Carver W., Kiaris H., Cui T., Tan W. The SARS-CoV-2 Spike protein induces long-term transcriptional perturbations of mitochondrial metabolic genes, causes cardiac fibrosis, and reduces myocardial contractile in obese mice. Mol. Metab. 2023;74:101756. doi: 10.1016/j.molmet.2023.101756. [PMC free article][PubMed] [CrossRef] [Google Scholar]
103. Baumeier C., Aleshcheva G., Harms D., Gross U., Hamm C., Assmus B., Westenfeld R., Kelm M., Rammos S., Wenzel P., et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int. J. Mol. Sci. 2022;23:6940. doi: 10.3390/ijms23136940. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
104. Barmada A., Klein J., Ramaswamy A., Brodsky N.N., Jaycox J.R., Sheikha H., Jones K.M., Habet V., Campbell M., Sumida T.S., et al. Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis. Sci. Immunol. 2023;8:eadh3455. doi: 10.1126/sciimmunol.adh3455. [PMC free article][PubMed] [CrossRef] [Google Scholar]
105. Wu C.T., Chin S.C., Chu P.H. Acute Fulminant Myocarditis After ChAdOx1 nCoV-19 Vaccine: A Case Report and Literature Review. Front. Cardiovasc. Med. 2022;9:856991. doi: 10.3389/fcvm.2022.856991. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
106. Sulemankhil I., Abdelrahman M., Negi S.I. Temporal Association Between the COVID-19 Ad26.COV2.S Vaccine and Acute Myocarditis: A Case Report and Literature Review. Cardiovasc. Revasc. Med. 2022;38:117–123. doi: 10.1016/j.carrev.2021.08.012. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
107. Olejniczak M., Schwartz M., Webber E., Shaffer A., Perry T.E. Viral Myocarditis-Incidence, Diagnosis and Management. J. Cardiothorac. Vasc. Anesth. 2020;34:1591–1601. doi: 10.1053/j.jvca.2019.12.052. [PubMed] [CrossRef] [Google Scholar]
108. Basso C. Myocarditis. N. Engl. J. Med. 2022;387:1488–1500. doi: 10.1056/NEJMra2114478. [PubMed] [CrossRef] [Google Scholar]
109. Simone A., Herald J., Chen A., Gulati N., Shen A.Y., Lewin B., Lee M.S. Acute Myocarditis Following COVID-19 mRNA Vaccination in Adults Aged 18 Years or Older. JAMA Intern. Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
110. Lane S., Yeomans A., Shakir S. Systematic review of spontaneous reports of myocarditis and pericarditis in transplant recipients and immunocompromised patients following COVID-19 mRNA vaccination. BMJ Open. 2022;12:e060425. doi: 10.1136/bmjopen-2021-060425. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
111. Naik R. FDA: Summary Basis for (…)ory Action. fda.gov. [(accessed on 8 November 2022)];2021 Available online: https://www.fda.gov/media/151733/download
112. Tschöpe C., Ammirati E., Bozkurt B., Caforio A.L.P., Cooper L.T., Felix S.B., Hare J.M., Heidecker B., Heymans S., Hübner N., et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
113. Mansanguan S., Charunwatthana P., Piyaphanee W., Dechkhajorn W., Poolcharoen A., Mansanguan C. Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Trop. Med. Infect. Dis. 2022;7:196. doi: 10.3390/tropicalmed7080196. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
114. Müller C., Buergin N., Lopez-Ayala P., Hirsiger J.R., Mueller P., Median D., Glarner N., Rumora K., Herrmann T., Koechlin L., et al. Sex-specific differences in myocardial injury incidence after COVID-19 mRNA-1273 Booster Vaccination. Eur. J. Heart Fail. 2023. accepted author manuscript . [PubMed] [CrossRef]
115. Manno E.C., Amodio D., Cotugno N., Rossetti C., Giancotta C., Santilli V., Zangari P., Rotulo G.A., Villani A., Giglioni E., et al. Higher Troponin Levels on Admission are associated With Persistent Cardiac Magnetic Resonance Lesions in Children Developing Myocarditis After mRNA-Based COVID-19 Vaccination. Pediatr. Infect. Dis. J. 2023;42:166–171. doi: 10.1097/INF.0000000000003762. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
116. Dowd E. Cause Unknown: The Epidemic of Sudden Deaths in 2021 and 2022. Children’s Health Defence; Washington, DC, USA: 2022. [Google Scholar]
117. Dowd E., Nunes Y., Alegria C. US Disability Data: Part 4—Relation with Excess Deaths. Bureau of Labor Statistics (BLS). Phinancetechnologies.com. 2022. [(accessed on 8 November 2022)]. Available online: https://phinancetechnologies.com/HumanityProjects/US%20Disabilities%20-%20Part4.htm
118. Therapeutic Goods Administration (TGA), FOI Reply 4093-02 Advisory Committee on Vaccines (ACV) Meeting 22, Minutes on item 2.1, BNT162b2 [mRNA] vaccine. [(accessed on 7 April 2023)];2021 Available online: https://www.tga.gov.au/sites/default/files/2023-03/foi-4093-02.pdf
119. Angeli F., Spanevello A., Reboldi G., Visca D., Verdecchia P. SARS-CoV-2 vaccines: Lights and shadows. Eur. J. Intern. Med. 2021;88:1–8. doi: 10.1016/j.ejim.2021.04.019. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
120. Angeli F., Reboldi G., Trapasso M., Zappa M., Spanevello A., Verdecchia P. COVID-19, vaccines and deficiency of ACE2 and other angiotensinases. Closing the loop on the “Spike effect” Eur. J. Intern. Med. 2022;103:23–28. doi: 10.1016/j.ejim.2022.06.015. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
121. Kuhn C.C., Basnet N., Bodakuntla S., Alvarez-Brecht P., Nichols S., Martinez-Sanchez A., Agostini L., Soh Y.M., Takagi J., Biertümpfel C., et al. Direct Cryo-ET observation of platelet deformation induced by SARS-CoV-2 spike protein. Nat. Commun. 2023;14:620. doi: 10.1038/s41467-023-36279-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
122. Zheng Y., Zhao J., Li J., Guo Z., Sheng J., Ye X., Jin G., Wang C., Chai W., Yan J., et al. SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate. Int. J. Biol. Macromol. 2021;193:1124–1129. doi: 10.1016/j.ijbiomac.2021.10.112. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
123. Ryu J.K., Sozmen E.G., Dixit K., Montano M., Matsui Y., Liu Y., Helmy E., Deerinck T.J., Yan Z., Schuck R., et al. SARS-CoV-2 spike protein induces abnormal inflammatory blood clots neutralized by fibrin immunotherapy. bioRxiv. 2021 doi: 10.1101/2021.10.12.464152. preprint . [CrossRef] [Google Scholar]
124. Boschi C., Scheim D.E., Bancod A., Militello M., Bideau M.L., Colson P., Fantini J., Scola B. SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects. Int. J. Mol. Sci. 2022;23:15480. doi: 10.3390/ijms232415480. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
125. Purnell M.C., Skrinjar T.J. Bioelectric Field Enhancement: The Influence on Membrane Potential and Cell Migration In Vitro. Adv. Wound Care. 2016;5:539–545. doi: 10.1089/wound.2016.0708. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
126. Purnell M.C., Skrinjar T.J. The dielectrophoretic disassociation of chloride ions and the influence on diamagnetic anisotropy in cell membranes. Discov. Med. 2016;22:257–273. [PubMed] [Google Scholar]
127. Papasimakis N., Fedotov V.A., Savinov V., Raybould T.A., Zheludev N.I. Electromagnetic toroidal excitations in matter and free space. Nat. Mater. 2016;15:263–271. doi: 10.1038/nmat4563. [PubMed] [CrossRef] [Google Scholar]
128. Purnell M.C., Butawan M.B.A., Ramsey R.D. Bio-field array: A dielectrophoretic electromagnetic toroidal excitation to restore and maintain the golden ratio in human erythrocytes. Physiol. Rep. 2018;6:e13722. doi: 10.14814/phy2.13722. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
129. Kooijman S., Meurs I., van der Stoep M., Habets K.L., Lammers B., Berbée J.F., Havekes L.M., van Eck M., Romijn J.A., Korporaal S.J., et al. Hematopoietic α7 nicotinic acetylcholine receptor deficiency increases inflammation and platelet activation status, but does not aggravate atherosclerosis. J. Thromb. Haemost. 2015;13:126–135. doi: 10.1111/jth.12765.[PubMed] [CrossRef] [Google Scholar]
130. Li J.X., Wang Y.H., Bair H., Hsu S.B., Chen C., Wei J.C., Lin C.J. Risk assessment of retinal vascular occlusion after COVID-19 vaccination. NPJ Vaccines. 2023;8:64. doi: 10.1038/s41541-023-00661-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
131. Herrera-Comoglio R., Lane S. Vaccine-Induced Immune Thrombocytopenia and Thrombosis after the Sputnik V Vaccine. N. Engl. J. Med. 2022;387:1431–1432. doi: 10.1056/NEJMc2210813. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
132. Buoninfante A., Andeweg A., Baker A.T., Borad M., Crawford N., Dogné J.M., Garcia-Azorin D., Greinacher A., Helfand R., Hviid A., et al. Understanding thrombosis with thrombocytopenia syndrome after COVID-19 vaccination. NPJ Vaccines. 2022;7:141. doi: 10.1038/s41541-022-00569-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
133. Leung H.H.L., Perdomo J., Ahmadi Z., Zhen S.S., Rashi F.N., Enjeti A., Ting S.B., Chong J.J.H., Chong B.H. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2022;13:5206. doi: 10.1038/s41467-022-32946-1. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
134. Greinacher A., Schönborn L., Siegerist F., Steil L., Palankar R., Handtke S., Reder A., Thiele T., Aurich K., Methling K., et al. Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT) Seminars Hematol. 2022;59:97–107. doi: 10.1053/j.seminhematol.2022.02.004. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
135. Talotta R., Robertson E.S. Antiphospholipid antibodies and risk of poast-COVID-19 vaccination thrombophilia: The straw that breaks the camels’s back? Cytokine Growth Factor Rev. 2021;60:52–60. doi: 10.1016/j.cytogfr.2021.05.001. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
136. Khavinson V., Terekhov A., Kormilets D., Maryanovich A. Homology between SARS CoV-2 and human proteins. Sci. Rep. 2021;11:17199. doi: 10.1038/s41598-021-96233-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
137. Kelleni M. SARS-CoV-2 vaccination, autoimmunity, antibody dependent Covid-19 enhancement and other potential risks: Beneath the tip of the iceberg. Int. J. Pulm. Respir. Sci. 2021;5:555658. doi: 10.19080/IJOPRS.2021.05.555658. [CrossRef] [Google Scholar]
138. Alqatari S., Ismail M., Hasan M., Bukhara R., Al Argan R., Alwaheed A., Alkhafaji D., Ahmed S., Hadhiah K., Alamri T., et al. Emergence of post COVID-19 vaccine autoimmune diseases: A single centre study. Infect. Drug Resist. 2023;16:1263–1278. doi: 10.2147/IDR.S394602. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
139. Rodriguez Y., Rojas M., Beltran S., Polo F., Camacho-Dominguez L., Morales S.D., Gershwin M.E., Anaya J.M. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J. Autoimmun. 2022;132:102898. doi: 10.1016/j.jaut.2022.102898. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
140. Lansang R.P., Amdemichael E., Sajic D. IgA pemphigus following COVID-19 vaccination: A case report. SAGE Open Med. Case Rep. 2023;11:2050313X231181022. doi: 10.1177/2050313X231181022. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
141. Minakawa S., Matsuzaki Y., Yao S., Sagara C., Akasaka E., Koga H., Ishii N., Hashimoto T., Sawamura D. Case report: A case of epidermolysis bullosa acquisita with IgG and IgM anti-basement membrane zone antibodies relapsed after COVID-19 mRNA vaccination. Front. Med. 2023;10:1093827. doi: 10.3389/fmed.2023.1093827. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
142. Makiyama A., Abe Y., Furusawa H., Kogami M., Ando T., Tada K., Onimaru M., Ishizu A., Yamaji K., Tamura N. Polyarteritis nodosa diagnosed in a young male after COVID-19 vaccine: A case report. Mod. Rheumatol. Case Rep. 2023 doi: 10.1093/mrcr/rxad037. [PubMed] [CrossRef] [Google Scholar]
143. Takedani K., Notsu M., Ishiai N., Asami Y., Uchida K., Kanasaki K. Graves’ disease after exposure to the SARS-CoV-2 vaccine: A case report and review of the literature. BMC Endocr. Disord. 2023;23:132. doi: 10.1186/s12902-023-01387-2.[PMC free article] [PubMed] [CrossRef] [Google Scholar]
144. Morimoto N., Mori T., Shioji S., Taguchi T., Watanabe H., Sakai K., Mori K., Yamamura A., Hanioka A., Akagi Y., et al. Rapidly progressive IgA nephropathy with membranoproliferative glomerulonephritis-like lesions in an elderly man following the third dose of an mRNA COVID-19 vaccine: A case report. BMC Nephrol. 2023;24:108. doi: 10.1186/s12882-023-03169-3.[PMC free article] [PubMed] [CrossRef] [Google Scholar]
145. Aochi S., Uehara M., Yamamoto M. IgG4-related Disease Emerging after COVID-19 mRNA Vaccination. Intern. Med. 2023;62:1547–1551. doi: 10.2169/internalmedicine.1125-22. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
146. Cam F., Gok G., Celiker H. Granulomatous anterior uveitis following mRNA-based COVID-19 vaccination: A case report. Indian J. Ophthalmol. 2023;71:1033–1035. doi: 10.4103/ijo.IJO_1771_22. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
147. Yamamoto M., Keino D., Sumii S., Yokosuka T., Goto H., Inui A., Sogo T., Kawakami M., Tanaka M., Yanagimachi M. Severe Hepatitis-associated Aplastic Anemia Following COVID-19 mRNA Vaccination. Intern. Med. 2023;62:1813–1816. doi: 10.2169/internalmedicine.1308-22. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
148. Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases” Clin. Immunol. 2021;224:108665. doi: 10.1016/j.clim.2021.108665. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
149. Irrgang P., Gerling J., Kocher K., Lapuente D., Steininger P., Habenicht K., Wytopil M., Beileke S., Schäfer S., Zhong J., et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023;8:eade2798. doi: 10.1126/sciimmunol.ade2798. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
150. Uversky V.N., Redwan E.M., Makis W., Rubio-Casillas A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines. 2023;11:991. doi: 10.3390/vaccines11050991.[PMC free article] [PubMed] [CrossRef] [Google Scholar]
151. Patel N.R., Anzalone M.L., Buja L.M., Elghetany M.T. Sudden cardiac death due to coronary artery involvement by IgG4-related disease: A rare, serious complication of a rare disease. Arch. Pathol. Lab. Med. 2014;138:833–836. doi: 10.5858/arpa.2012-0614-CR. [PubMed] [CrossRef] [Google Scholar]
152. Gutierrez P.S., Schultz T., Siqueira S.A., de Figueiredo Borges L. Sudden coronary death due to IgG4-related disease. Cardiovasc. Pathol. 2013;22:505–507. doi: 10.1016/j.carpath.2013.05.003. [PubMed] [CrossRef] [Google Scholar]
153. Martín-Nares E., Saavedra-González V., Fagundo-Sierra R., Santinelli-Núñez B.E., Romero-Maceda T., Calderón-Vasquez K., Hernandez-Molina G. Serum immunoglobulin free light chains and their association with clinical phenotypes, serology and activity in patients with IgG4-related disease. Sci. Rep. 2021;11:1832. doi: 10.1038/s41598-021-81321-5. [PMC free article][PubMed] [CrossRef] [Google Scholar]
154. Tsai H.C., Tung H.Y., Liu C.W., Su C.F., Sun Y.S., Chen W.S., Chen M.H., Lai C.C., Liao H.T., Yang Y.Y., et al. Significance of high serum IgG4 in complete or non-full-fledged IgG4-related disease-a retrospective investigation of 845 patients and its clinical relevance. Clin. Rheumatol. 2022;41:115–122. doi: 10.1007/s10067-021-05772-x. [PubMed] [CrossRef] [Google Scholar]
155. Campochiaro C., Ramirez G.A., Bozzolo E.P., Lanzillotta M., Berti A., Baldissera E., Dagna L., Praderio L., Scotti R., Tresoldi M., et al. IgG4-related disease in Italy: Clinical features and outcomes of a large cohort of patients. Scand. J. Rheumatol. 2016;45:135–145. doi: 10.3109/03009742.2015.1055796. [PubMed] [CrossRef] [Google Scholar]
156. Wallace Z.S., Zhang Y., Perugino C.A., Naden R., Choi H.K., Stone J.H., ACR/EULAR IgG4-RD Classification Criteria Committee Clinical phenotypes of IgG4-related disease: An analysis of two international cross-sectional cohorts. Ann. Rheum. Dis. 2019;78:406–412. doi: 10.1136/annrheumdis-2018-214603. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
157. Chen L.Y.C., Mattman A., Seidman M.A., Carruthers M.N. IgG4-related disease: What a hematologist needs to know. Haematologica. 2019;104:444–455. doi: 10.3324/haematol.2018.205526. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
158. Lin W., Lu S., Chen H., Wu Q., Fei Y., Li M., Zhang X., Tian X., Zheng W., Leng X., et al. Clinical characteristics of immunoglobulin G4-related disease: A prospective study of 118 Chinese patients. Rheumatology. 2015;54:1982–1990. doi: 10.1093/rheumatology/kev203. [PubMed] [CrossRef] [Google Scholar]
159. Della-Torre E., Lanzillotta M., Doglioni C. Immunology of IgG4-related disease. Clin. Exp. Immunol. 2015;181:191–206. doi: 10.1111/cei.12641. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
160. Stone J.R. Aortitis, periaortitis, and retroperitoneal fibrosis, as manifestations of IgG4-related systemic disease. Curr. Opin. Rheumatol. 2011;23:88–94. doi: 10.1097/BOR.0b013e3283412f7c. [PubMed] [CrossRef] [Google Scholar]
161. Prapruttam D., Hedgire S.S., Mani S.E., Chandramohan A., Shyamkumar N.K., Harisinghani M. Tuberculosis–the great mimicker. Semin. Ultrasound CT MR. 2014;35:195–214. doi: 10.1053/j.sult.2014.02.002. [PubMed] [CrossRef] [Google Scholar]
162. (PHMPT) Public Health & Medical Professionals for Transparency, Pfizer’s Documents. 2022. [(accessed on 10 July 2023)]. Available online: https://phmpt.org/
163. Pfizer. 5.3.6 Cumulative Analysis of Post-Authorization of Adverse Event Reports of PF-07302048 (BNT162B2) Received through 28-Feb-2021. FDA-CBER-2021-5683-0000054. Public Health and Medical Professionals for Transparency (PHMPT) [(accessed on 14 July 2023)]. Available online: https://phmpt.org/pfizer-16-plus-documents/
164. Nuovo G.J., Suster D., Sawant D., Mishra A., Michaille J.J., Tili E. The amplification of CNS damage in Alzheimer’s disease due to SARS-CoV2 infection. Ann. Diagn. Pathol. 2022;61:152057. doi: 10.1016/j.anndiagpath.2022.152057. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
165. Rastogi A., Bingeliene A., Strafella A.P., Tang-Wai D.F., Wu P.E., Mandell D.M. Reversible neurological and brain MRI changes following COVID-19 vaccination: A case report. J. Neuroradiol. 2022;49:428–430. doi: 10.1016/j.neurad.2022.03.011. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
166. Rhea E.M., Logsdon A.F., Hansen K.M., Williams L.M., Reed M.J., Baumann K.K., Holden S.J., Raber J., Banks W.A., Erickson M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021;24:368–378. doi: 10.1038/s41593-020-00771-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
167. Mörz M. A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines. 2022;10:1651. doi: 10.3390/vaccines10101651. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
168. Kim E.S., Jeon M.T., Kim K.S., Lee S., Kim S., Kim D.G. Spike Proteins of SARS-CoV-2 Induce Pathological Changes in Molecular Delivery and Metabolic Function in the Brain Endothelial Cells. Viruses. 2021;13:2021. doi: 10.3390/v13102021.[PMC free article] [PubMed] [CrossRef] [Google Scholar]
169. Khaddaj-Mallat R., Aldib N., Bernard M., Paquette A.S., Ferreira A., Lecordier S., Saghatelyan A., Flamand L., ElAli A. SARS-CoV-2 deregulates the vascular and immune functions of brain pericytes via Spike protein. Neurobiol. Dis. 2021;161:105561. doi: 10.1016/j.nbd.2021.105561. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
170. Fontes-Dantas F.L., Fernandes G.G., Gutman E.G., De Lima E.V., Antonio L.S., Hammerle M.B., Mota-Araujo H.P., Colodeti L.C., Araújo S.M.B., Froz G.M., et al. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 2023;42:112189. doi: 10.1016/j.celrep.2023.112189.[PMC free article] [PubMed] [CrossRef] [Google Scholar]
171. Oh J., Cho W.H., Barcelon E., Kim K.H., Hong J., Lee S.J. SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci. Rep. 2022;12:5496. doi: 10.1038/s41598-022-09410-7. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
172. Tillman T.S., Chen Q., Bondarenko V., Coleman J.A., Xu Y., Tang P. SARS-CoV-2 Spike Protein Downregulates Cell Surface alpha7nAChR through a Helical Motif in the Spike Neck. ACS Chem. Neurosci. 2023;14:689–698. doi: 10.1021/acschemneuro.2c00610. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
173. Rong Z., Mai H., Kapoor S., Puelles V.G., Czogalla J., Schädler J., Vering J., Delbridge C., Steinke H., Frenzel H., et al. SARS-CoV-2 Spike Protein Accumulation in the Skull-Meninges-Brain Axis: Potential Implications for Long-Term Neurological Complications in post-COVID-19. bioRxiv. 2023:preprint. doi: 10.1101/2023.04.04.535604. [CrossRef] [Google Scholar]
174. Olajide O.A., Iwuanyanwu V.U., Adegbola O.D., Al-Hindawi A.A. SARS-CoV-2 Spike Glycoprotein S1 Induces Neuroinflammation in BV-2 Microglia. Mol. Neurobiol. 2022;59:445–458. doi: 10.1007/s12035-021-02593-6. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
175. Wu Z., Zhang X., Huang Z., Ma K. SARS-CoV-2 Proteins Interact with Alpha Synuclein and Induce Lewy Body-like Pathology In Vitro. Int. J. Mol. Sci. 2022;23:3394. doi: 10.3390/ijms23063394. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
176. Winkler E.S., Bailey A.L., Kafai N.M., Sharmila N., McCune B.T., Jinsheng Y., Fox J.M., Chen R.E., Earnest J.J., Keeler S.P., et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020;21:1327–1335. doi: 10.1038/s41590-020-0778-2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
177. Lykhmus O., Kalashnyk O., Skok M. Positive Allosteric Modulation of Alpha7 Nicotinic Acetylcholine Receptors Transiently Improves Memory but Aggravates Inflammation in LPS-Treated Mice. Front. Aging Neurosci. 2020;11:359. doi: 10.3389/fnagi.2019.00359. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
178. Lykhmus O., Kalashnyk O., Koval L., Krynina O., Komisarenko S., Skok M. Immunization with 674–685 fragment of SARS-Cov-2 spike protein induces neuroinflammation and impairs episodic memory of mice. Biochem. Biophys. Res. Commun. 2022;622:57–63. doi: 10.1016/j.bbrc.2022.07.016. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
179. Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N., Ding Z., Eggel A., et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
180. Fernández-Castañeda A., Lu P., Geraghty A.C., Song E., Lee M.H., Wood J., O’Dea M.R., Dutton S., Shamardani K., Nwangwu K., et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–2468.e16. doi: 10.1016/j.cell.2022.06.008. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
181. Shrestha N.K., Burke P.C., Nowacki A.S., Simon J.F., Hagen A., Gordon S.M. Effectiveness of the Coronavirus Disease 2019 Bivalent Vaccine. Open Forum Infect. Dis. 2023;10:ofad209. doi: 10.1093/ofid/ofad209. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
182. Coleman J.M., Naik C., Holguin F., Ray A., Ray P., Trudeau J.B., Wenzel S.E. Epithelial eotaxin-2 and eotaxin-3 expression: Relation to asthma severity, luminal eosinophilia and age at onset. Thorax. 2012;67:1061–1066. doi: 10.1136/thoraxjnl-2012-201634. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
183. Rojas-Ramos E., Avalos A.F., Perez-Fernandez L., Cuevas-Schacht F., Valencia-Maqueda E., Teran L.M. Role of the chemokines RANTES, monocyte chemotactic proteins-3 and −4, and eotaxins-1 and −2 in childhood asthma. Eur. Respir. J. 2003;22:310–316. doi: 10.1183/09031936.03.00084802. [PubMed] [CrossRef] [Google Scholar]
184. Holgate S.T. Mucosal Immunology. Academic Press; Cambridge, MA, USA: 2015. [CrossRef] [Google Scholar]
185. Nyström S., Hammarström P. Amyloidogenesis of SARS-CoV-2 Spike Protein. J. Am. Chem. Soc. 2022;144:8945–8950. doi: 10.1021/jacs.2c03925. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
186. Maatuk N., Samson A.O. Modeling the binding mechanism of Alzheimer’s Aβ1-42 to nicotinic acetylcholine receptors based on similarity with snake α-neurotoxins. Neurotoxicology. 2013;34:236–242. doi: 10.1016/j.neuro.2012.09.007.[PubMed] [CrossRef] [Google Scholar]
187. Lasala M., Fabiani C., Corradi J., Antollini S., Bouzat C. Molecular Modulation of Human α7 Nicotinic Receptor by Amyloid-β Peptides. Front. Cell. Neurosci. 2019;13:37. doi: 10.3389/fncel.2019.00037. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
188. Dhakal S., Wyant C.E., George H.E., Morgan S.E., Rangachari V. Prion-like C-Terminal Domain of TDP-43 and α-Synuclein Interact Synergistically to Generate Neurotoxic Hybrid Fibrils. J. Mol. Biol. 2021;433:166953. doi: 10.1016/j.jmb.2021.166953. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
189. Tetz G., Tetz V. Prion-like Domains in Spike Protein of SARS-CoV-2 Differ across Its Variants and Enable Changes in Affinity to ACE2. Microorganisms. 2022;10:280. doi: 10.3390/microorganisms10020280. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
190. Nonaka T., Hasegawa M. TDP-43 Prions. Cold Spring Harb. Perspect. Med. 2018;8:a024463. doi: 10.1101/cshperspect.a024463. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
191. Classen J. COVID-19 RNA based vaccines and the risk of prion disease. Microbiol. Infect. Dis. 2021;5:1–3. doi: 10.33425/2639-9458.1109. [CrossRef] [Google Scholar]
192. Idrees D., Kumar V. SARS-CoV-2 spike protein interactions with amyloidogenic proteins: Potential clues to neurodegeneration. Biochem. Biophys. Res. Commun. 2021;554:94–98. doi: 10.1016/j.bbrc.2021.03.100. [PMC free article][PubMed] [CrossRef] [Google Scholar]
193. Kuvandik A., Özcan E., Serin S., Sungurtekin H. Creutzfeldt-Jakob Disease After the COVID-19 Vaccination. Turk. J. Intensive Care. 2021;20:61–64. doi: 10.4274/tybd.galenos.2021.91885. [CrossRef] [Google Scholar]
194. Wang F., Wang X., Yuan C.G., Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
195. Young M.J., O’Hare M., Matiello M., Schmahmann J.D. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav. Immun. 2020;89:601–603. doi: 10.1016/j.bbi.2020.07.007. [PMC free article][PubMed] [CrossRef] [Google Scholar]
196. d’Errico P., Meyer-Luehmann M. Mechanisms of Pathogenic Tau and Abeta Protein Spreading in Alzheimer’s Disease. Front. Aging Neurosci. 2020;12:265. doi: 10.3389/fnagi.2020.00265. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
197. Duda J.E., Lee V.M., Trojanowski J.Q. Neuropathology of synuclein aggregates. J. Neurosci. Res. 2000;61:121–127. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. [PubMed] [CrossRef] [Google Scholar]
198. Stefano G.B. Historical Insight into Infections and Disorders Associated with Neurological and Psychiatric Sequelae Similar to Long COVID. Med. Sci. Monit. 2021;27:e931447. doi: 10.12659/MSM.931447. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
199. MedAlerts.org This is VAERS ID 1754471. National Vaccine Information Center: 2021. [(accessed on 4 July 2023)]. Available online: https://medalerts.org/vaersdb/findfield.php
200. MedAlerts.org This is VAERS ID 1777781. National Vaccine Information Center: 2021. [(accessed on 4 July 2023)]. Available online: https://medalerts.org/vaersdb/findfield.php
201. Australian Bureau of Statistics (ABS) Provisional Mortality Statistics: Reference Period: Jan—Dec 2022; abs.gov.au, 2023. [(accessed on 7 July 2023)]; Available online: https://www.abs.gov.au/statistics/health/causes-death/provisional-mortality-statistics/jan-dec-2022
202. Australian Bureau of Statistics (ABS) Provisional Mortality Statistics. Australian Bureau of Statistics (ABS); Canberra, Australia: 2023. [Google Scholar]
203. Kuo P.H., Chiang C.H., Wang Y.T., Doudeva L.G., Yuan H.S. The crystal structure of TDP-43 RRM1-DNA complex reveals the specific recognition for UG- and TG-rich nucleic acids. Nucleic Acids Res. 2014;42:4712–4722. doi: 10.1093/nar/gkt1407. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
204. King O.D., Gitler A.D., Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
205. Tavassoly O., Safavi F., Tavassoly I. Seeding Brain Protein Aggregation by SARS-CoV-2 as a Possible Long-Term Complication of COVID-19 Infection. ACS Chem. Neurosci. 2020;11:3704–3706. doi: 10.1021/acschemneuro.0c00676.[PubMed] [CrossRef] [Google Scholar]
206. Mueller B.K., Subramaniam S., Senes A. A frequent, GxxxG-mediated, transmembrane association motif is optimized for the formation of interhelical Calpha-H hydrogen bonds. Proc. Natl. Acad. Sci. USA. 2014;111:E888–E895. doi: 10.1073/pnas.1319944111. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
207. Prusiner S.B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [PubMed] [CrossRef] [Google Scholar]
208. Decock M., Stanga S., Octave J.N., Dewachter I., Smith S.O., Constantinescu S.N., Kienlen-Campard P. Glycines from the APP GXXXG/GXXXA Transmembrane Motifs Promote Formation of Pathogenic Abeta Oligomers in Cells. Front. Aging Neurosci. 2016;8:107. doi: 10.3389/fnagi.2016.00107. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
209. Seneff S., Nigh G. Worse Than the Disease? Reviewing Some Possible Unintended Consequences of the mRNA Vaccines Against COVID-19. Int. J. Vaccine Theory Pract. Res. 2021;2:38–79. doi: 10.56098/ijvtpr.v2i1.23. [CrossRef] [Google Scholar]
210. Liu-Yesucevitz L., Bilgutay A., Zhang Y.J., Vanderweyde T., Citro A., Mehta T., Zaarur N., McKee A., Bowser R., Sherman M., et al. Tar DNA binding protein-43 (TDP-43) associates with stress granules: Analysis of cultured cells and pathological brain tissue. PLoS ONE. 2010;5:e13250. doi: 10.1371/journal.pone.0013250. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
211. Bosco D.A., Lemay N., Ko H.K., Zhou H., Burke C., Kwiatkowski T.J., Jr., Sapp P., McKenna-Yasek D., Brown R.H., Jr., Hayward L.J. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet. 2010;19:4160–4175. doi: 10.1093/hmg/ddq335. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
212. Cox P.A., Richer R., Metcalf J.S., Banack S.A., Codd G.A., Bradley W.G. Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lateral Scler. 2009;10((Suppl S2)):109–117. doi: 10.3109/17482960903286066. [PubMed] [CrossRef] [Google Scholar]
213. Stefano G.B., Buttiker P., Weissenberger S., Anders M., Raboch J., Ptacek R., Kream R.M. Potential Prion Involvement in Long COVID-19 Neuropathology, Including Behavior. Cell. Mol. Neurobiol. 2023;43:2621–2626. doi: 10.1007/s10571-023-01342-8. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
214. Donaldson D.S., Bradford B.M., Else K.J., Mabbott N.A. Accelerated onset of CNS prion disease in mice co-infected with a gastrointestinal helminth pathogen during the preclinical phase. Sci. Rep. 2020;10:4554. doi: 10.1038/s41598-020-61483-4.[PMC free article] [PubMed] [CrossRef] [Google Scholar]
215. Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Munch A.E., Chung W.S., Peterson T.C., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
216. Makarava N., Chang J.C., Molesworth K., Baskakov I.V. Region-specific glial homeostatic signature in prion diseases is replaced by a uniform neuroinflammation signature, common for brain regions and prion strains with different cell tropism. Neurobiol. Dis. 2020;137:104783. doi: 10.1016/j.nbd.2020.104783. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
217. Xu J., Wei H., You P., Sui J., Xiu J., Zhu W., Xu Q. Non-neutralizing antibodies to SARS-Cov-2-related linear epitopes induce psychotic-like behavior in mice. Front. Mol. Neurosci. 2023;16:1177961. doi: 10.3389/fnmol.2023.1177961. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
218. Kyriakopoulos A.M., Nigh G., McCullough P.A., Seneff S. Mitogen Activated Protein Kinase (MAPK) Activation, p53, and Autophagy Inhibition Characterize the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Protein Induced Neurotoxicity. Cureus. 2022;14:e32361. doi: 10.7759/cureus.32361. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
219. Thomas C.A., Paquola A.C.M., Muotri A.R. LINE-1 retrotransposition in the nervous system. Annu. Rev. Cell Dev. Biol. 2012;28:555–573. doi: 10.1146/annurev-cellbio-101011-155822. [PubMed] [CrossRef] [Google Scholar]
220. Terry D.M., Devine S.E. Aberrantly High Levels of Somatic LINE-1 Expression and Retrotransposition in Human Neurological Disorders. Front. Genet. 2020;10:1244. doi: 10.3389/fgene.2019.01244. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
221. Shamila D., Alipoor S.D., Moratz E., Garssen J., Movassaghi M., Mirsaeidi M., Adcock I.M. Exosomes and Exosomal miRNA in Respiratory Diseases. Mediat. Inflamm. 2016;2016:5628404. doi: 10.1155/2016/5628404. [PMC free article][PubMed] [CrossRef] [Google Scholar]
222. Visacri M.B., Nicoletti A.S., Pincinato E.C., Loren P., Saavedra N., Saavedra K., Salazar L.A., Moriel P. Role of miRNAs as biomarkers of COVID-19: A scoping review of the status and future directions for research in this field. Biomark. Med. 2021;15:1785–1795. doi: 10.2217/bmm-2021-0348. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
223. Pogue A.I., Lukiw W.J. microRNA-146a-5p, Neurotropic Viral Infection and Prion Disease (PrD) Int. J. Mol. Sci. 2021;22:9198. doi: 10.3390/ijms22179198. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
224. Lukiw W.J., Dua P., Pogue A.I., Eicken C., Hill J.M. Upregulation of micro RNA-146a (miRNA-146a), a marker for inflammatory neurodegeneration, in sporadic Creutzfeldt-Jakob disease (sCJD) and Gerstmann-Straussler-Scheinker (GSS) syndrome. J. Toxicol. Environ. Health A. 2011;74:1460–1468. doi: 10.1080/15287394.2011.618973. [PMC free article][PubMed] [CrossRef] [Google Scholar]
225. Norrby E. Prions and protein-folding diseases. J. Intern. Med. 2011;270:1–14. doi: 10.1111/j.1365-2796.2011.02387.x.[PubMed] [CrossRef] [Google Scholar]
226. Horwich A.L., Weissman J.S. Deadly conformations—Protein misfolding in prion disease. Cell. 1997;89:499–510. doi: 10.1016/S0092-8674(00)80232-9. [PubMed] [CrossRef] [Google Scholar]
227. Tripathi U., Nchioua R., Prata L.G.P.L., Zhu Y., Gerdes E.O.W., Giorgadze N., Pirtskhalava T., Parker E., Xue A., Espindola-Netto J.M., et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging. 2021;13:21838–21854. doi: 10.18632/aging.203560. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
228. Sfera A., Thomas K., Sfera D., Anton J., Andronescu C., Jafri N., Susanna S., Kozlakidis Z. Do messenger RNA vaccines induce pathological syncytial? Int. J. Pathol. Clin. Res. 2022;8:137. [Google Scholar]
229. Huang L., Jin R., Li J., Luo K., Huang T., Wu D., Wang W., Chen R., Xiao G. Macromolecular crowding converts the human recombinant PrPC to the soluble neurotoxic beta-oligomers. FASEB J. 2010;24:3536–3543. doi: 10.1096/fj.09-150987.[PubMed] [CrossRef] [Google Scholar]
230. Dalgleish A. Interview with Professor Angus Dalgleish. Immunotherapy. 2016;8:1271–1276. doi: 10.2217/imt-2016-0120. [PubMed] [CrossRef] [Google Scholar]
231. Dalgleish A. As an Oncologist I am Seeing People with Stable Cancer Rapidly Progress after Being Forced to Have a Booster. Letter to Dr Abbassi, Editor-in-Chief BMJ. dailysceptic.org. 2022. [(accessed on 11 June 2023)]. Available online: https://dailysceptic.org/2022/11/26/as-an-oncologist-i-am-seeing-people-with-stable-cancer-rapidly-progress-after-being-forced-to-have-a-booster/
232. Pio R., Ajona D., Ortiz-Espinosa S., Mantovani A., Lambris J.D. Complementing the cancer-immunity cycle. Front. Immunol. 2019;10:774. doi: 10.3389/fimmu.2019.00774. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
233. Alsaab H.O., Sau S., Alzhrani R., Tatiparti K., Bhise K., Kashaw S.K., Lyer A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
234. Bishawi M., Bowles D., Pla M.M., Oakes F., Chiang Y.S., Schroder J., Milano C., Glass C. PD-1 and PD-L1 expression in cardiac transplantation. Cardiovasc. Pathol. 2021;54:107331. doi: 10.1016/j.carpath.2021.107331. [PubMed] [CrossRef] [Google Scholar]
235. Loacker L., Kimpel J., Bánki Z., Schmidt C.Q., Griesmacher A., Anliker M. Increased PD-L1 surface expression on peripheral blood granulocytes and monocytes after vaccination with SARS-CoV2 mRNA or vector vaccine. Clin. Chem. Lab. Med. 2022;61:e17–e19. doi: 10.1515/cclm-2022-0787. [PubMed] [CrossRef] [Google Scholar]
236. Diskin C., Ryan T.A.J., O’Neill L.J. Modification of Proteins by Metabolites in Immunity. Immunity. 2021;54:19–31. doi: 10.1016/j.immuni.2020.09.014. [PubMed] [CrossRef] [Google Scholar]
237. Mishra R., Banerjea A.C. SARS-CoV-2 Spike targets USP33-IRF9 axis via exosomal miR-148a to activate human microglia. Front. Immunol. 2021;12:656700. doi: 10.3389/fimmu.2021.656700. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
238. Seneff S., Nigh G., Kyriakopoulos A.M., McCoulough P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022;164:113008. doi: 10.1016/j.fct.2022.113008. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
239. Hofer M.J., Li W., Lim S.L., Campbell I.L. The type I interferon-alpha mediates a more severe neurological disease in the absence of the canonical signaling molecule interferon regulatory factor 9. J. Neurosci. 2010;30:1149–1157. doi: 10.1523/JNEUROSCI.3711-09.2010. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
240. Rasmussen S.A., Abul-Husn N.S., Casanova J.L., Daly M.J., Rehm H.L., Murray M.F. The intersection of genetics and COVID-19 in 2021: Preview of the 2021 Rodney Howell Symposium. Genet. Med. 2021;23:1001–1003. doi: 10.1038/s41436-021-01113-0. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
241. Liu J., Wang J., Xu J., Xia H., Wang Y., Zhang C., Chen W., Zhang H., Liu Q., Zhu R., et al. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell Discov. 2021;7:99. doi: 10.1038/s41421-021-00329-3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
242. Li S., Silvestri V., Leslie G., Rebbeck T.R., Neuhausen S.L., Hopper J.L., Nielsen H.R., Lee A., Yang X., McGuffog L., et al. Cancer Risks Associated With BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022;40:1529–1541. doi: 10.1200/JCO.21.02112. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
243. Nuovo G.J., Magro C., Shaffer T., Awad H., Suster D., Mikhail S., He B., Michaille J.J., Liechty B., Tili E. Endothelial cell damage is the central part of COVID-19 and a mouse model induced by injection of the S1 subunit of the spike protein. Ann. Diagn. Pathol. 2021;51:151682. doi: 10.1016/j.anndiagpath.2020.151682. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
244. Mezache L., Nuovo G.J., Suster D., Tili E., Awad H., Radwański P.B., Veeraraghavan R. Histologic, viral, and molecular correlates of heart disease in fatal COVID-19. Ann. Diagn. Pathol. 2022;60:151983. doi: 10.1016/j.anndiagpath.2022.151983.[PMC free article] [PubMed] [CrossRef] [Google Scholar]
245. Choi J. Fauci: Amount of Virus in ‘Breakthrough Delta Cases Almost Identical’ to Unvaccinated. The Hill: Thehill.com. 2021. [(accessed on 7 April 2023)]. Available online: https://thehill.com/homenews/sunday-talk-shows/565831-fauci-amount-of-virus-in-breakthrough-delta-cases-almost-identical/
246. Schwab C., Domke L.M., Hartmann L., Stenzinger A., Longerich T., Schirmacher P. Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination. Clin. Res. Cardiol. 2023;112:431–440. doi: 10.1007/s00392-022-02129-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
247. Burkhardt A. Reutlingen Autopsy/Histology Study: Side-Effects from Corona Vaccinations. PowerPoint Conference Presentation (in German). Corona-blog.net. 2022. [(accessed on 7 April 2023)]. Available online: https://corona-blog.net/2022/03/10/reutlinger-autopsie-histologie-studie-nebenwirkungen-und-todesfaelle-durch-die-corona-impfungen/
248. Burkhardt A. Pathology Conference: Vaccine-Induced Spike Protein Production in the Brain, Organs etc., now Proven. Report24.news. 2022. [(accessed on 7 April 2023)]. Available online: https://report24.news/pathologie-konferenz-impfinduzierte-spike-produktion-in-gehirn-u-a-organen-nun-erwiesen/
249. Domazet-Lošo T. mRNA Vaccines: Why Is the Biology of Retroposition Ignored? Genes. 2022;13:719. doi: 10.3390/genes13050719. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
250. Dopp K., Seneff S. COVID-19 and all-cause mortality data by age group reveals risk of COVID vaccine-induced fatality is equal to or greater than the risk of a COVID death for all age groups under 80 years old as of 6 February 2022. Vixra.org. 2022;21:preprint. [Google Scholar]
251. Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2020;11:571416. doi: 10.3389/fphys.2020.571416. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
252. Chen Y., Li C., Liu F., Ye Z., Song W., Lee A.C.Y., Shuai H., Lu L., To K.K., Chan J.F., et al. Age-associated SARS-CoV-2 breakthrough infection and changes in immune response in a mouse model. Emerg. Microbes Infect. 2022;11:368–383. doi: 10.1080/22221751.2022.2026741. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
253. Vo A.D., La J., Wu J.T., Strymish J.M., Ronan M., Brophy M., Do N.V., Branch-Elliman W., Fillmore N.R., Monach P.A. Factors Associated With Severe COVID-19 Among Vaccinated Adults Treated in US Veterans Affairs Hospitals. JAMA Netw. Open. 2022;5:e2240037. doi: 10.1001/jamanetworkopen.2022.40037. [PMC free article] [PubMed] [CrossRef] [Google Scholar]

















